Betri ea lithium-ion kapa betri ea Li-ion (e khutsufalitsoeng e le LIB) ke mofuta oa betri e ka tjhajoang hape.Libetri tsa lithium-ion li atisa ho sebelisoa bakeng sa likoloi tse nkehang tsa elektronike le likoloi tsa motlakase 'me li ntse li hōla ka botumo bakeng sa lisebelisoa tsa sesole le tsa sefofane.Betri ea mohlala ea Li-ion e entsoe ke Akira Yoshino ka 1985, e ipapisitse le liphuputso tsa pejana tse entsoeng ke John Goodenough, M. Stanley Whittingham, Rachid Yazami le Koichi Mizushima ka bo-1970-1980s, 'me betri ea Li-ion ea khoebo Sehlopha sa Sony le Asahi Kasei se etelletsoeng pele ke Yoshio Nishi ka 1991. Ka 2019, Moputso oa Nobel oa Chemistry o ile oa fuoa Yoshino, Goodenough, le Whittingham "bakeng sa nts'etsopele ea libeteri tsa lithium ion".
Ka libeteri, li-ion tsa lithium li tloha ho electrode e mpe ka electrolyte ho ea ho electrode e ntle nakong ea ho tsoa, le ho khutlela morao ha e tjhaja.Li-betri tsa Li-ion li sebelisa motsoako oa lithium o kopantsoeng e le lisebelisoa ho electrode e ntle 'me hangata ke graphite ho electrode e mpe.Libetri li na le matla a mangata a matla, ha ho na mohopolo (ntle le lisele tsa LFP) le ho itšehla thajana.Leha ho le joalo e ka ba kotsi ea polokeho kaha li na le li-electrolyte tse tukang, 'me haeba li senyehile kapa li qosoa ka phoso li ka baka ho phatloha le mollo.Samsung e ile ea qobelloa ho hopola li-handsets tsa Galaxy Note 7 ka mor'a mollo oa lithium-ion, 'me ho bile le liketsahalo tse' maloa tse amanang le libeteri tsa Boeing 787s.
Litšobotsi tsa k'hemistri, ts'ebetso, litšenyehelo le polokeho li fapana ho mefuta ea LIB.Lisebelisoa tsa elektronike tse tšoaroang ka letsoho hangata li sebelisa libeteri tsa lithium polymer (tse nang le gel ea polymer e le electrolyte) e nang le lithium cobalt oxide (LiCoO2) e le thepa ea cathode, e fanang ka matla a phahameng a matla, empa e fana ka likotsi tsa polokeho, haholo-holo ha e senyehile.Lithium iron phosphate (LiFePO4), lithium manganese oxide (LiMn2O4, Li2MnO3, kapa LMO), le lithium nickel manganese cobalt oxide (LiNiMnCoO2 kapa NMC) li fana ka matla a fokolang a matla empa bophelo bo bolelele le monyetla o fokolang oa mollo kapa ho phatloha.Libetri tse joalo li sebelisoa haholo bakeng sa lisebelisoa tsa motlakase, lisebelisoa tsa bongaka le mesebetsi e meng.NMC le lihlahisoa tsa eona li sebelisoa haholo likoloing tsa motlakase.
Libaka tsa lipatlisiso bakeng sa libeteri tsa lithium-ion li kenyelletsa ho lelefatsa bophelo, ho eketsa matla a matla, ho ntlafatsa polokeho, ho fokotsa litšenyehelo, le ho eketsa lebelo la ho tjhaja, har'a tse ling.Lipatlisiso li ntse li tsoela pele sebakeng sa li-electrolyte tse sa cheng e le tsela ea ho eketsa polokeho e thehiloeng ho chefo le ho feto-fetoha ha metsoako ea tlhaho e sebelisoang ho electrolyte e tloaelehileng.Maano a kenyelletsa libeteri tsa lithium-ion tse nang le metsi, li-electrolyte tse tiileng tsa ceramic, li-electrolyte tsa polymer, metsi a ionic, le lisebelisoa tse nang le fluorinated haholo.
Betri khahlano le sele
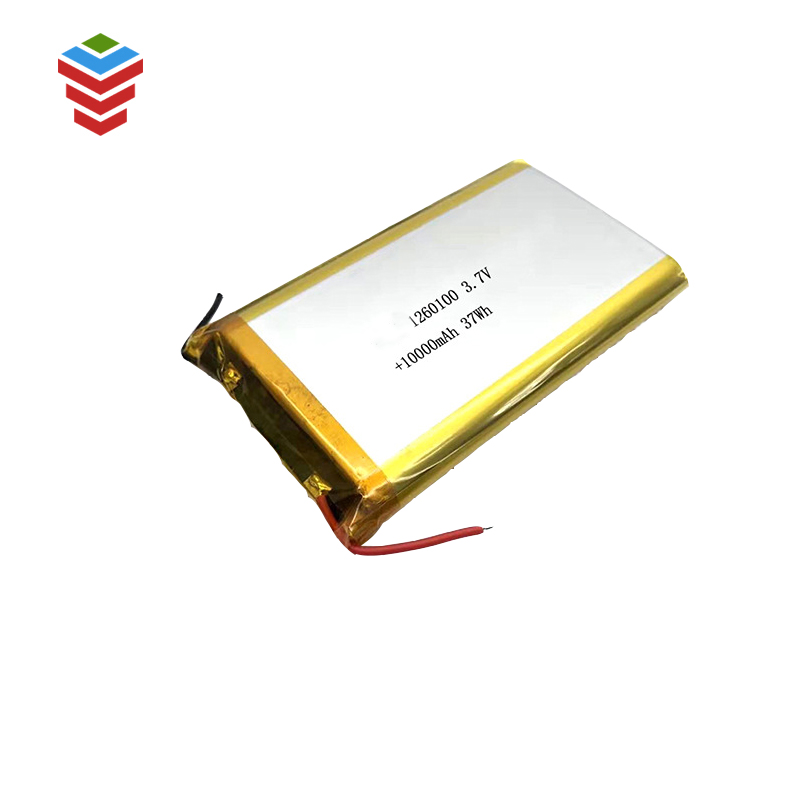

Sele ke setsi sa motheo sa electrochemical se nang le li-electrode, separator le electrolyte.
Betri kapa betri pakete ke pokello ea lisele kapa likopano tsa lisele, tse nang le matlo, likhokahano tsa motlakase, mohlomong le lisebelisoa tsa elektroniki bakeng sa taolo le ts'ireletso.
Li-electrode tsa anode le cathode
Bakeng sa lisele tse nchafatsoang, lentsoe anode (kapa electrode e fosahetseng) le bolela electrode moo oxidation e etsahalang nakong ea potoloho ea ho tsoa;electrode e 'ngoe ke cathode (kapa electrode e ntle).Nakong ea potoloho ea tefiso, electrode e ntle e fetoha anode le electrode e mpe e fetoha cathode.Bakeng sa lisele tse ngata tsa lithium-ion, electrode ea lithium-oxide ke electrode e ntle;bakeng sa lisele tsa lithium-ion tsa titanate (LTO), electrode ea lithium-oxide ke electrode e mpe.
Histori
Ka morao
Varta lithium-ion battery, Museum Autovision, Altlussheim, Jeremane
Libetri tsa Lithium li hlahisitsoe ke setsebi sa k'hemistri sa Brithani le moamoheli-'moho le Moputso oa Nobel oa 2019 bakeng sa k'hemistri M. Stanley Whittingham, eo hona joale a leng Univesithing ea Binghamton, ha a ntse a sebeletsa Exxon ka bo-1970.Whittingham o sebelisitse titanium(IV) sulfide le lithium metal e le li-electrode.Leha ho le joalo, betri ena ea lithium e ka nchafatsoang e ne e ke ke ea etsoa e sebetsang.Titanium disulfide e ne e le khetho e mpe, kaha e tlameha ho etsoa tlas'a maemo a tiisitsoeng ka ho feletseng, hape e theko e boima haholo (~$1,000 ka kilogram bakeng sa thepa e tala ea titanium disulfide ka bo-1970).Ha e le moeeng, titanium disulfide e arabela ho etsa metsoako ea hydrogen sulfide, e nang le monko o sa thabiseng le e chefo ho liphoofolo tse ngata.Ka lebaka lena, le mabaka a mang, Exxon o ile a khaotsa ho hlahisa betri ea Whittingham ea lithium-titanium disulfide.[28]Libetri tse nang le li-electrode tsa tšepe tsa lithium li hlahisitse litaba tsa polokeho, joalo ka ha tšepe ea lithium e kopana le metsi, e ntša khase ea haedrojene e tukang.Ka lebaka leo, lipatlisiso li ile tsa fallela ho hlahisa libeteri tseo ho tsona, ho e-na le lithium ea tšepe, ho na le metsoako ea lithium feela, e khonang ho amohela le ho lokolla li-ion tsa lithium.
Khokahano e fetolehang ho graphite le ho hokahana ho li-cathodic oxides e ile ea sibolloa nakong ea 1974-76 ke JO Besenhard ho TU Munich.Besenhard o khothalelitse ts'ebeliso ea eona liseleng tsa lithium.Ho bola ha elektrolyte le ho kopanngoa ha solvent ho graphite e bile litšitiso tse kholo tsa bophelo ba betri.
Tsoelo-pele
1973 - Adam Heller o ile a etsa tlhahiso ea betri ea lithium thionyl chloride, e ntseng e sebelisoa lisebelisoa tsa bongaka tse kentsoeng le lits'ebetsong tsa ts'ireletso moo ho hlokahalang nako e fetang lilemo tse 20, matla a mangata a matla, le / kapa mamello bakeng sa mocheso o feteletseng oa ho sebetsa.
1977 - Samar Basu o bontšitse tšebelisano ea electrochemical ea lithium ka graphite Univesithing ea Pennsylvania.Sena se ile sa lebisa ho nts'etsopele ea motlakase oa lithium intercalated graphite electrode ho Bell Labs (LiC6) ho fana ka mokhoa o mong oa betri ea lithium metal electrode.
1979 - Ba sebetsa ka lihlopha tse arohaneng, Ned A. Godshall et al., 'me, nakoana ka mor'a moo, John B. Goodenough (Univesithi ea Oxford) le Koichi Mizushima (Yunivesithing ea Tokyo), ba ile ba bontša sele ea lithium e ka nchafatsoang e nang le motlakase sebakeng sa 4 V ho sebelisa lithium. cobalt dioxide (LiCoO2) e le electrode e ntle le tšepe ea lithium e le electrode e mpe.Tlhahiso ena e fane ka lisebelisoa tse ntle tsa electrode tse nolofalitseng libeteri tsa pele tsa khoebo tsa lithium.LiCoO2 ke lintho tse tsitsitseng tse ntle tsa electrode tse sebetsang e le mofani oa li-ion tsa lithium, tse bolelang hore li ka sebelisoa ka lisebelisoa tse mpe tsa electrode ntle le tšepe ea lithium.Ka ho nolofalletsa tšebeliso ea lisebelisoa tsa li-electrode tse tsitsitseng le tse bonolo ho sebetsana le tsona, LiCoO2 e nolofalitse lisebelisoa tsa betri tse nchafalitsoeng.Molimo et al.ho feta moo ho ile ha supa boleng bo tšoanang ba ternary compound lithium-transition metal-oxides tse kang spinel LiMn2O4, Li2MnO3, LiMnO2, LiFeO2, LiFe5O8, le LiFe5O4 (le hamorao lithium-copper-oxide le lithium-nickel-oxide cathode materials ka 1985)
1980 - Rachid Yazami o ile a bontša phetoho ea electrochemical intercalation ea lithium ka graphite, 'me a qapa electrode ea lithium graphite (anode).Li-electrolyte tse fumanehang ka nako eo li ne li tla bola nakong ea ho tjhaja ka electrode e mpe ea graphite.Yazami o sebelisitse electrolyte e tiileng ho bonts'a hore lithium e ka boela ea kopanngoa ka graphite ka mochine oa electrochemical.Ho tloha ka 2011, electrode ea graphite ea Yazami e ne e le electrode e sebelisoang ka ho fetisisa libeteri tsa lithium-ion tsa khoebo.
Electrode e mpe e simolohile ho PAS (polyacenic semiconductive material) e fumanoeng ke Tokio Yamabe 'me hamorao ke Shjzukuni Yata mathoasong a lilemo tsa bo-1980.Peo ea theknoloji ena e ne e le ho sibolloa ha li-polymers tsa conductive ke Moprofesa Hideki Shirakawa le sehlopha sa hae, 'me e ka boela ea bonoa e qalile ho tloha polyacetylene lithium ion betri e entsoeng ke Alan MacDiarmid le Alan J. Heeger et al.
1982 - Godshall et al.ba ile ba fuoa US Patent 4,340,652 bakeng sa tšebeliso ea LiCoO2 e le cathodes ka libeteri tsa lithium, tse thehiloeng ho Godshall's Stanford University Ph.D.dissertation le likhatiso tsa 1979.
1983 - Michael M. Thackeray, Peter Bruce, William David, le John Goodenough ba ile ba hlahisa mokokotlo oa manganese e le thepa ea cathode e sebelisoang khoebong bakeng sa libeteri tsa lithium-ion.
1985 - Akira Yoshino o ile a bokella sele ea prototype e sebelisang thepa ea carbonaceous eo li-ion tsa lithium li ka kenngoa e le electrode e le 'ngoe, le lithium cobalt oxide (LiCoO2) e le e' ngoe.Sena se ile sa ntlafatsa tšireletseho ka tsela e hlollang.LiCoO2 e nolofalitse tlhahiso ea maemo a indasteri mme ea nolofalletsa betri ea lithium-ion ea khoebo.
1989 - Arumugam Manthiram le John B. Goodenough ba ile ba sibolla sehlopha sa polyanion sa cathodes.Ba bontšitse hore li-electrode tse ntle tse nang le li-polyanion, mohlala, li-sulfate, li hlahisa li-voltage tse phahameng ho feta li-oxide ka lebaka la phello ea inductive ea polyanion.Sehlopha sena sa polyanion se na le lisebelisoa tse kang lithium iron phosphate.
<e tla tswelapele…>
Nako ea poso: Mar-17-2021





